Guilford Techno Consultants, Inc
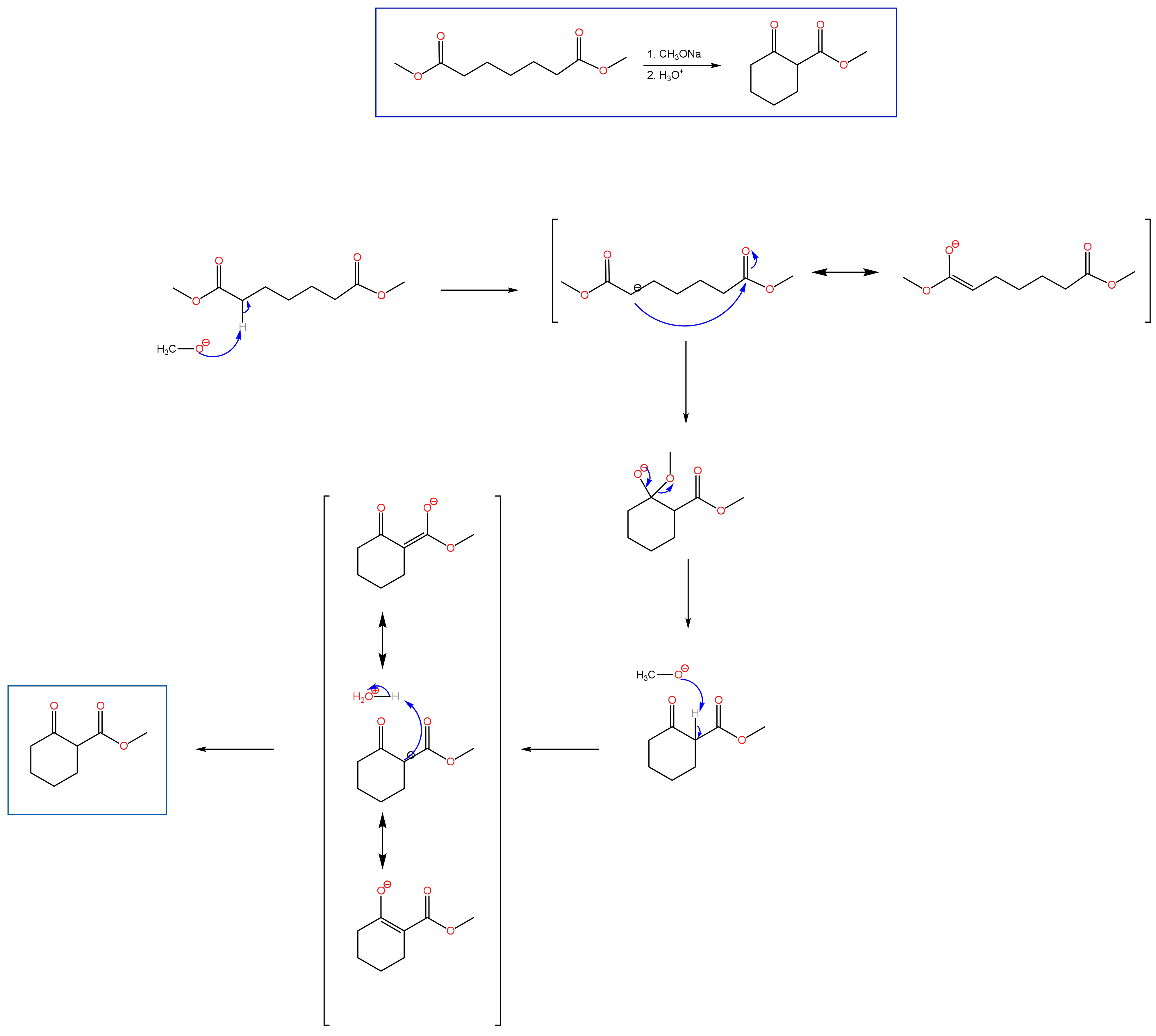
Claisen Condensation
Wednesday, April 19, 2023 by Guilford Techno Consultants, Inc. | Enolates
Carbonyl chemistry is a prominent part of the second semester of Organic Chemistry. An important component of carbonyl chemistry is reactions at the alpha carbon, such as the Aldol condensation that I spoke about in a previous post. Another condensation reaction covered in the second semester is the Claisen condensation. While an Aldol condensation is a reaction between aldehydes or ketones, the Claisen condensation involves the reaction of an ester with the enolate of another ester, a ketone, or aldehyde. If two esters are used as starting material, the product will be a β-keto ester. The resulting product from the condensation of an ester with a ketone will be a β-diketone. As with the Aldol condensation, the Claisen condensation can be performed with two moles of the same ester (self condensation). Unlike the Aldol condensation, the Claisen condensation involves two steps, the first being the generation of the enolate under basic conditions, followed by the subsequent attack of the second carbonyl compound to yield the product. After formation of the product, however, an acid step is needed to protonate the resulting alpha carbon between the two carbonyls. Below you will find the mechanism for the Dieckmann cyclization, which is an intramolecular Claisen condensation. Practice problems can be found under the OChem II tab.
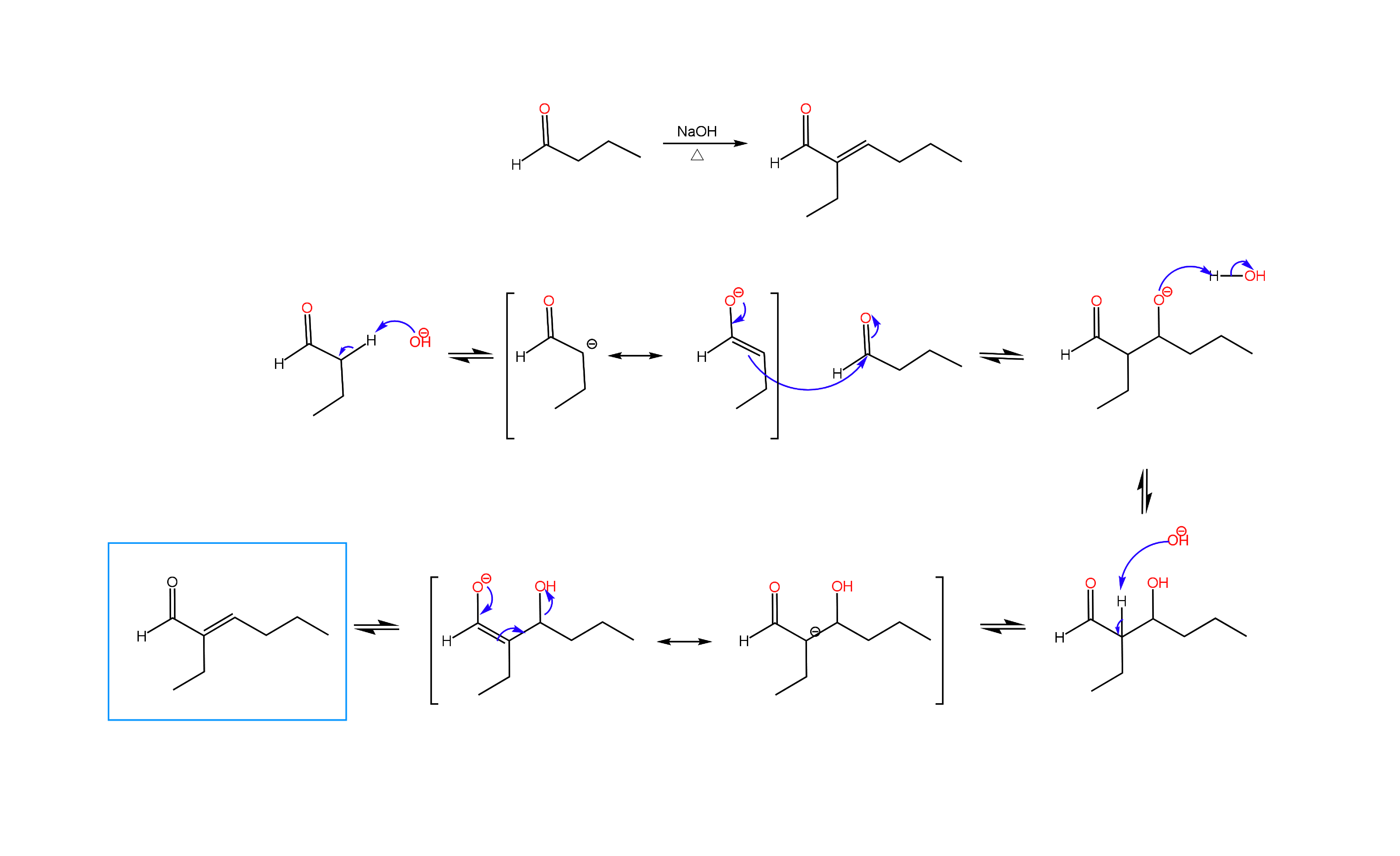
Aldol Condensation
Wednesday, March 29, 2023 by Guilford Techno Consultants, Inc. | Enolates
We are more than half way through the semester, and many OChem II students are learning about alpha carbon chemistry, the many reactions that can be done with enolates. One of the reactions that students find challenging is the Aldol condensation. In an Aldol condensation performed under basic conditions, an aldehyde or ketone is reacted with the enolate of another aldehyde or ketone, producing an α,β-unsaturated carbonyl compound. The reaction can be performed with two moles of the same molecule (self condensation) or one mole each of different molecules (mixed Aldol). In either case, the enolate of one molecule is formed through deprotonation of an alpha carbon with base, followed by addition of this enolate to the carbonyl of the second molecule to produce an α,β-hydroxy carbonyl compound (Aldol product). Elimination of the beta hydroxy group yields the α,β-unsaturated carbonyl compound (Condensation product). Below you will find an example of a self condensation of an aldehyde. Practice problems can be found under the OChem II tab.