Guilford Techno Consultants, Inc
E2: Beta Elimination
Sunday, April 30, 2023 by Guilford Techno Consultants, Inc. | Substitution and Elimination Reactions
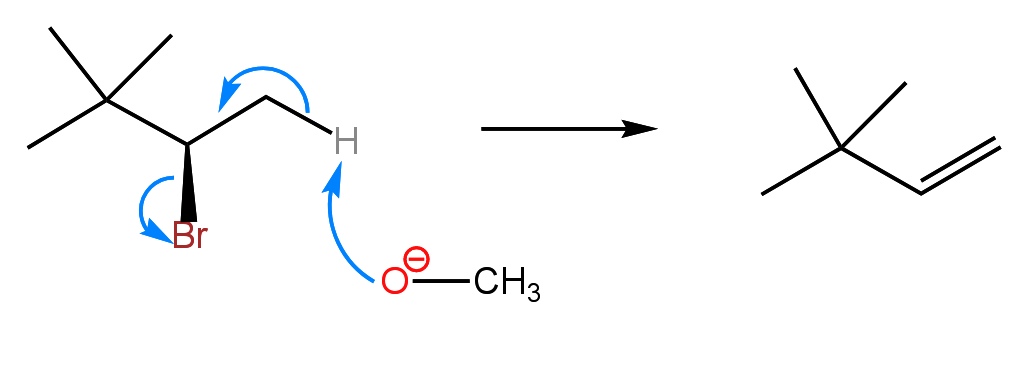
Most first semester students find substitution and elimination reactions to be overwhelming, in part because of the sheer volume of information needed to understand these reactions. In terms of mechanism, they are relatively simple in comparison to many of the other reactions of the course. Some of the difficulty stems from the fact that substitution and elimination reactions compete with each other, making product prediction a challenge. Many students have trouble distinguishing between a nucleophile and a base, and additionally determining what constitutes a strong or weak nucleophile or base. In E2 elimination, also known as beta elimination, a strong base (i.e., hydroxide, methoxide, t-butoxide) deprotonates a carbon that is beta (next to) a carbon with a leaving group (alpha carbon). The electrons from the C-H bond push towards the carbon with the leaving group, thus forming a C-C double bond with loss of the leaving group. Below is the mechanism for an E2 reaction.
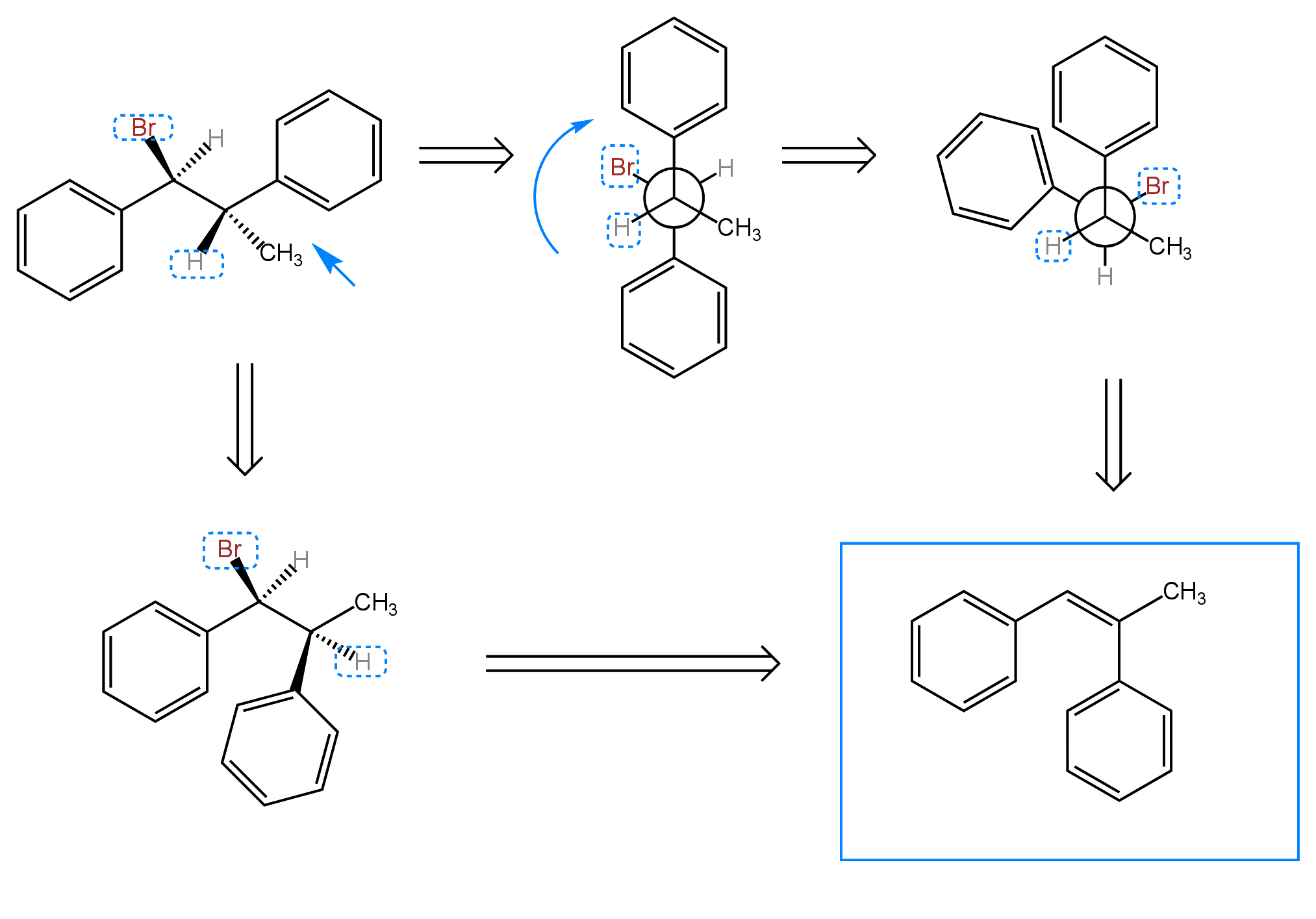
One of the problem spots of the E2 reaction is the requirement that the proton that is removed from the beta carbon is antiperiplanar to the leaving group, meaning that the proton and the leaving group must be roughly 180° apart. If this is not the case, the bond between the alpha and beta carbons must be rotated to meet this requirement. This requirement determines the stereochemical outcome (cis or trans) of the product. When I have to do a bond rotation to get the leaving group and the proton antiperiplanar to each other, I like to use a Newman projection. You can also do the bond rotation without a Newman projection if you prefer. Below, I illustrate both methods. Practice problems can be found under the OChemI tab. For each reaction, predict all of the possible products from E2 elimination.
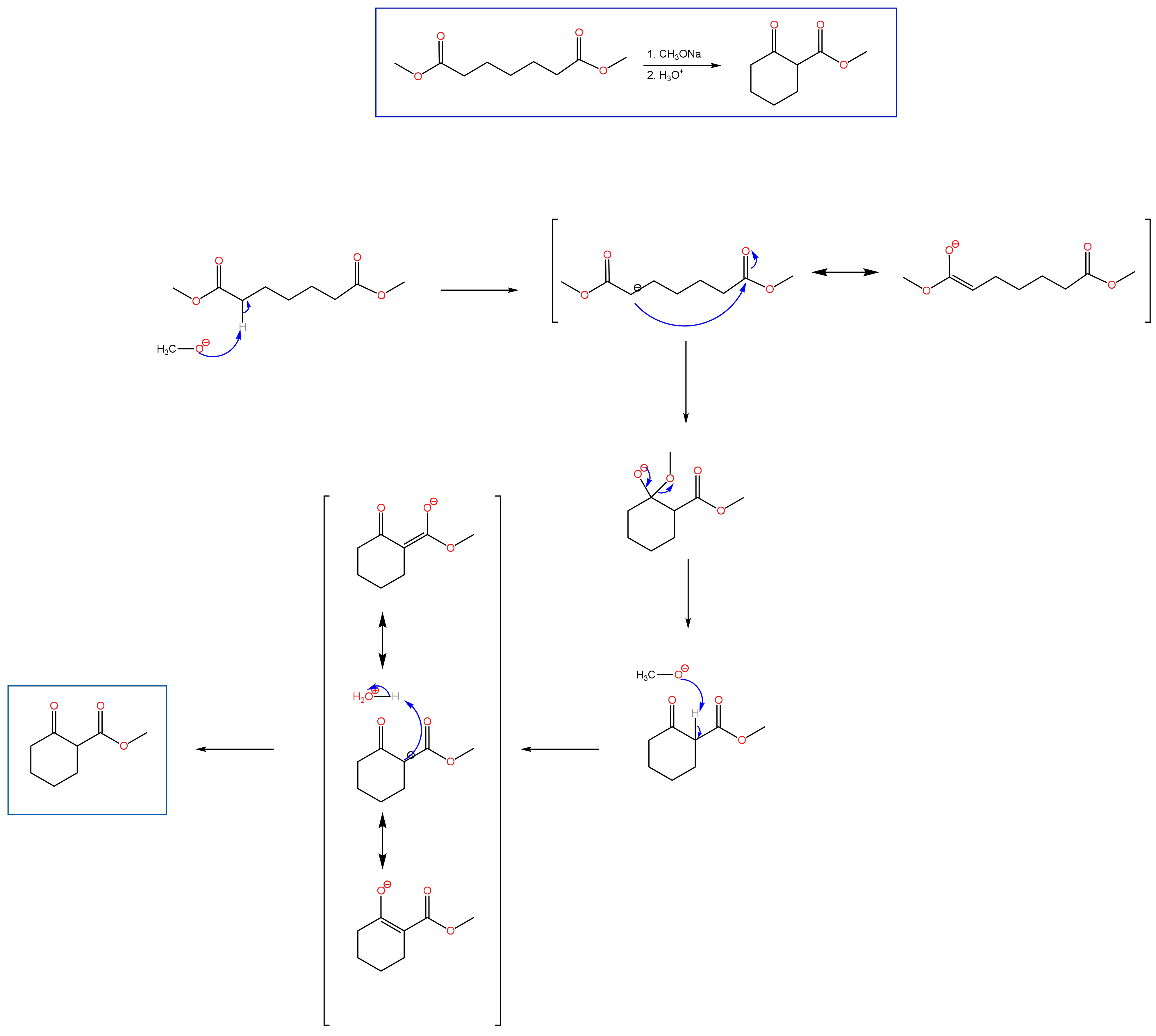
Claisen Condensation
Wednesday, April 19, 2023 by Guilford Techno Consultants, Inc. | Enolates
Carbonyl chemistry is a prominent part of the second semester of Organic Chemistry. An important component of carbonyl chemistry is reactions at the alpha carbon, such as the Aldol condensation that I spoke about in a previous post. Another condensation reaction covered in the second semester is the Claisen condensation. While an Aldol condensation is a reaction between aldehydes or ketones, the Claisen condensation involves the reaction of an ester with the enolate of another ester, a ketone, or aldehyde. If two esters are used as starting material, the product will be a β-keto ester. The resulting product from the condensation of an ester with a ketone will be a β-diketone. As with the Aldol condensation, the Claisen condensation can be performed with two moles of the same ester (self condensation). Unlike the Aldol condensation, the Claisen condensation involves two steps, the first being the generation of the enolate under basic conditions, followed by the subsequent attack of the second carbonyl compound to yield the product. After formation of the product, however, an acid step is needed to protonate the resulting alpha carbon between the two carbonyls. Below you will find the mechanism for the Dieckmann cyclization, which is an intramolecular Claisen condensation. Practice problems can be found under the OChem II tab.