Guilford Techno Consultants, Inc
Aldol Condensation
Wednesday, March 29, 2023 by Guilford Techno Consultants, Inc. | Enolates
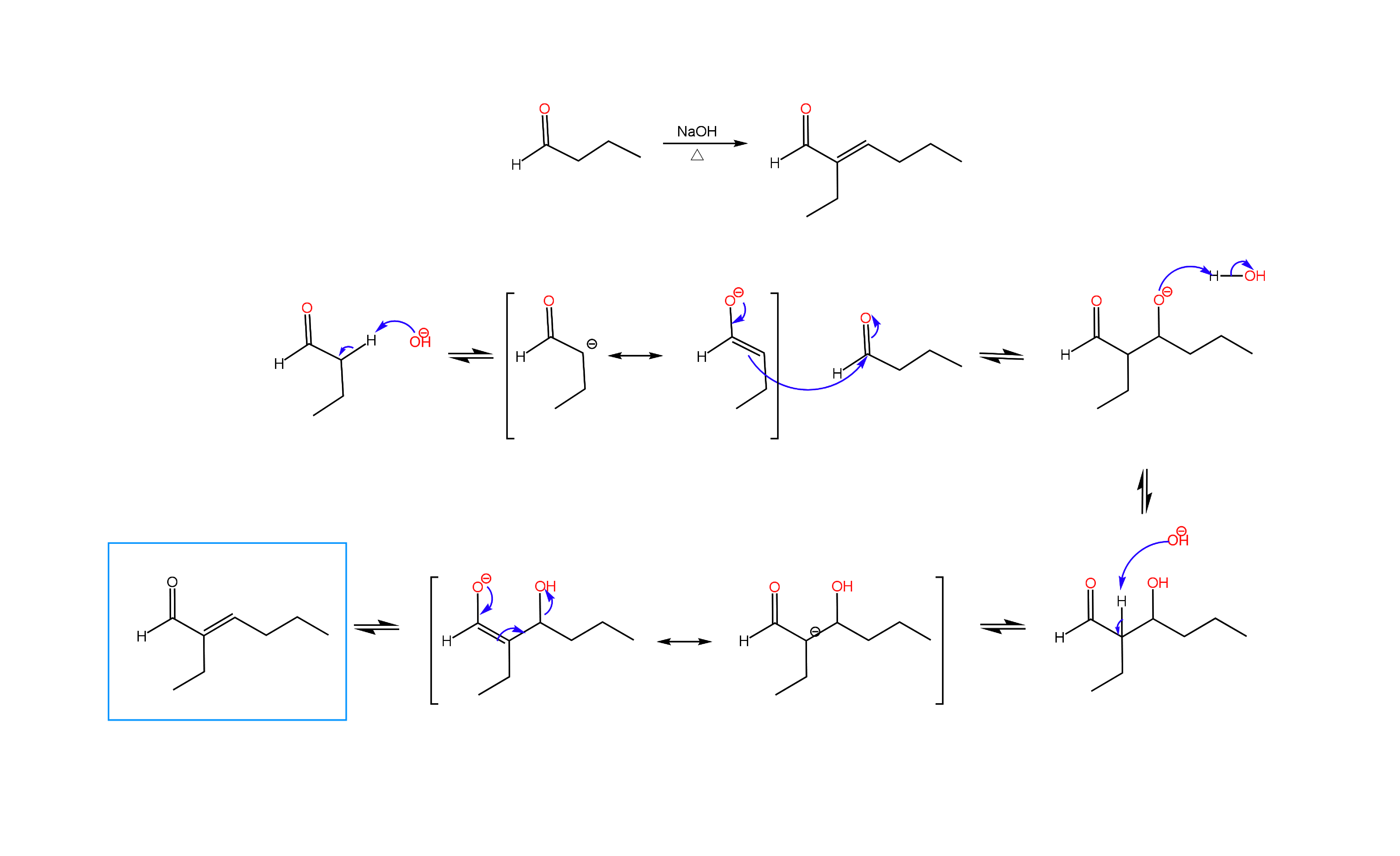
We are more than half way through the semester, and many OChem II students are learning about alpha carbon chemistry, the many reactions that can be done with enolates. One of the reactions that students find challenging is the Aldol condensation. In an Aldol condensation performed under basic conditions, an aldehyde or ketone is reacted with the enolate of another aldehyde or ketone, producing an α,β-unsaturated carbonyl compound. The reaction can be performed with two moles of the same molecule (self condensation) or one mole each of different molecules (mixed Aldol). In either case, the enolate of one molecule is formed through deprotonation of an alpha carbon with base, followed by addition of this enolate to the carbonyl of the second molecule to produce an α,β-hydroxy carbonyl compound (Aldol product). Elimination of the beta hydroxy group yields the α,β-unsaturated carbonyl compound (Condensation product). Below you will find an example of a self condensation of an aldehyde. Practice problems can be found under the OChem II tab.