Guilford Techno Consultants, Inc
Baeyer-Villiger Oxidation
Sunday, August 20, 2023 by Guilford Techno Consultants, Inc. | Name Reactions
Several mechanisms of the second semester of Organic Chemistry involve the migration (shift) of an alkyl group to give a rearranged product. The Hofmann rearrangement, which I wrote about in my last post, is one such mechanism. Another reaction that proceeds through an alkyl shift is the Baeyer-Villiger oxidation of a ketone by a peroxyacid to yield an ester. The reaction was discovered by Adolf von Baeyer and Victor Villiger in 1899 and involves the insertion of an oxygen atom of the peroxyacid in between the carbonyl of the ketone and one of it’s alkyl groups:
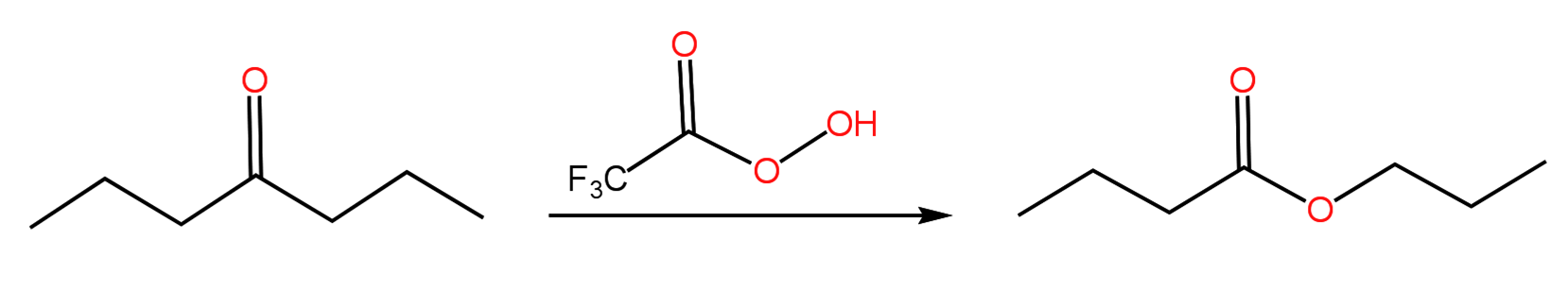
If the ketone is cyclic, a lactone (cyclic ester) is formed:
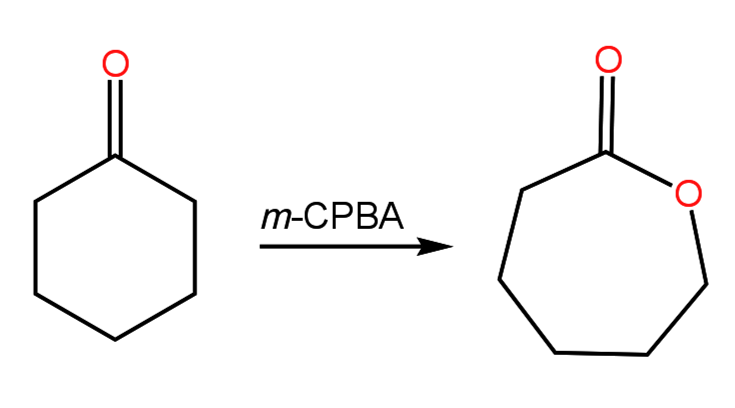
The mechanism involves the formation of an uncharged tetrahedral intermediate after attack of the protonated carbonyl by the peroxyacid. The carbonyl is reformed through migration of an alkyl group and subsequent deprotonation. The peroxyacid is converted to a carboxylic acid during the process. If the ketone is asymmetric, the reaction is regiospecific with respect to which group will migrate:
H > 3°C > 2°C, phenyl > 1°C > CH3
In other words, hydrogen will migrate faster than any other group, while methyl will migrate slower than any other group. The mechanism can be found below, along with practice problems.
Hofmann Rearrangement
Thursday, August 3, 2023 by Guilford Techno Consultants, Inc. | Name Reactions
The topic of amines is covered in the second semester of Organic Chemistry. In addition to learning about the physical properties, spectroscopy, and reactivity of amines, students also learn about the ways to prepare them. Of course, threaded throughout the material are the various mechanisms associated with the reactions. To me, amine mechanism is in a category by itself. I don’t find mechanisms involving amines to be very intuitive and consequently, I find them to be the some of the most challenging of the two semesters.
The Hofmann rearrangement, discovered by August Wilhelm von Hofmann, is a mechanism that many students find particularly challenging. The reaction involves the conversion of a primary amide to a primary amine with the production of carbon dioxide as a side product:
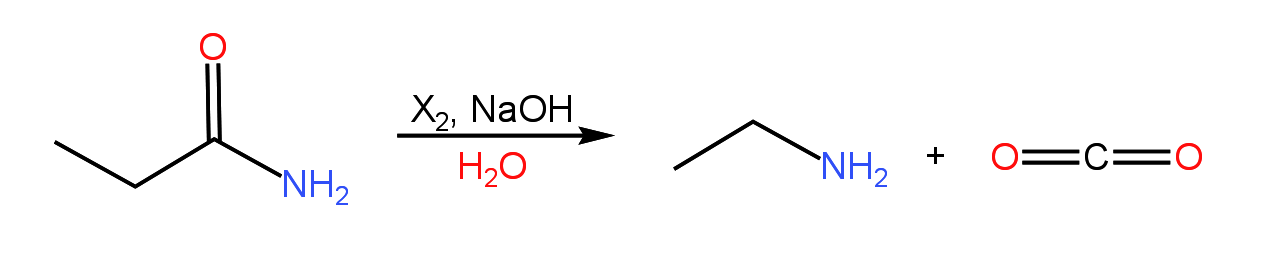
There are two mechanistic pieces to the reaction. The first involves a rearrangement of an N-haloamidate to an isocyante, while the second involves decarboxylation of a carbamate to produce the primary amine. Hydroxide begins the reaction by deprotonating the amide nitrogen to produce an amidate ion. The nitrogen of the amidate ion is subsequently halogenated by X2. Following this step, hydroxide removes a proton from the nitrogen, yielding an N-haloamidate. A shift of the alkyl group next the carbonyl results in rearrangement to an isocyanate with loss of a halide ion. Under the basic conditions of the reaction, the isocyanate spontaneously hydrates to form a carbamate ion. Loss of CO2, followed by protonation of the nitrogen, yields the primary amine. Reaction of hydroxide with CO2 produces bicarbonate, driving the reaction to completion by preventing the amine from reacting with CO2. A complete mechanism and practice problems can be found in the following presentation.
Mitsunobu Reaction
Wednesday, August 2, 2023 by Guilford Techno Consultants, Inc. | Name Reactions
The Mitsunobu reaction, discovered by Oyo Mitsunobu, is a reaction in which a primary or secondary alcohol can be reacted with a nucleophile through an SN2 mechanism. The reaction is unique in that the hydroxyl group of the alcohol is converted into a better leaving group, and the resulting electrophilic species is subsequently attacked by the negatively charged nucleophile in a “one pot” synthesis. In other words, the conversion of the hydroxyl to a better leaving group and the subsequent nucleophilic attack take place in one reaction mixture, unlike other nucleophilic displacements of the hydroxyl group that involve two separate steps (i.e., tosylate formation).
In addition to the alcohol, there are three other reactants involved in the reaction; the nucleophile, triphenyl phosphine, and diethylazodicarboxylate (DEAD). A requirement of the nucleophile is that it has an acidic proton. Appropriate nucleophiles for the reaction include carboxylic acids, thiols, and phenols, to name a few. The anion of the nucleophile is produced through deprotonation. Triphenyl phosphine serves to convert the alcohol to the electrophilic species. Both the deprotonation of the nucleophile and conversion of the alcohol to the electrophile are facilitated by DEAD, which has several roles in the mechanism. The side products of the reaction are triphenyl phosphine oxide and the reduced form of DEAD. As the mechanism is SN2, inversion of stereochemistry of chiral secondary alcohols is observed. The mechanism for the reaction along with practice problems can be found in the following presentation.
Click Here for the Mitsunobu Reaction

