Guilford Techno Consultants, Inc
Hofmann Rearrangement
Thursday, August 3, 2023 by Guilford Techno Consultants, Inc. | Name Reactions
The topic of amines is covered in the second semester of Organic Chemistry. In addition to learning about the physical properties, spectroscopy, and reactivity of amines, students also learn about the ways to prepare them. Of course, threaded throughout the material are the various mechanisms associated with the reactions. To me, amine mechanism is in a category by itself. I don’t find mechanisms involving amines to be very intuitive and consequently, I find them to be the some of the most challenging of the two semesters.
The Hofmann rearrangement, discovered by August Wilhelm von Hofmann, is a mechanism that many students find particularly challenging. The reaction involves the conversion of a primary amide to a primary amine with the production of carbon dioxide as a side product:
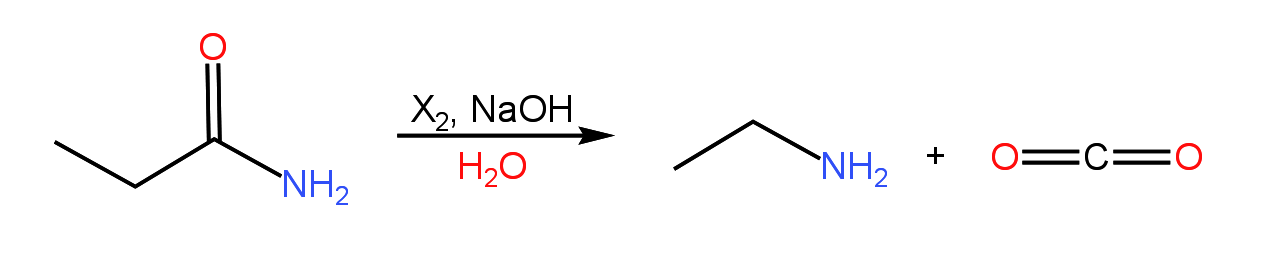
There are two mechanistic pieces to the reaction. The first involves a rearrangement of an N-haloamidate to an isocyante, while the second involves decarboxylation of a carbamate to produce the primary amine. Hydroxide begins the reaction by deprotonating the amide nitrogen to produce an amidate ion. The nitrogen of the amidate ion is subsequently halogenated by X2. Following this step, hydroxide removes a proton from the nitrogen, yielding an N-haloamidate. A shift of the alkyl group next the carbonyl results in rearrangement to an isocyanate with loss of a halide ion. Under the basic conditions of the reaction, the isocyanate spontaneously hydrates to form a carbamate ion. Loss of CO2, followed by protonation of the nitrogen, yields the primary amine. Reaction of hydroxide with CO2 produces bicarbonate, driving the reaction to completion by preventing the amine from reacting with CO2. A complete mechanism and practice problems can be found in the following presentation.

