Guilford Techno Consultants, Inc
Baeyer-Villiger Oxidation
Sunday, August 20, 2023 by Guilford Techno Consultants, Inc. | Name Reactions
Several mechanisms of the second semester of Organic Chemistry involve the migration (shift) of an alkyl group to give a rearranged product. The Hofmann rearrangement, which I wrote about in my last post, is one such mechanism. Another reaction that proceeds through an alkyl shift is the Baeyer-Villiger oxidation of a ketone by a peroxyacid to yield an ester. The reaction was discovered by Adolf von Baeyer and Victor Villiger in 1899 and involves the insertion of an oxygen atom of the peroxyacid in between the carbonyl of the ketone and one of it’s alkyl groups:
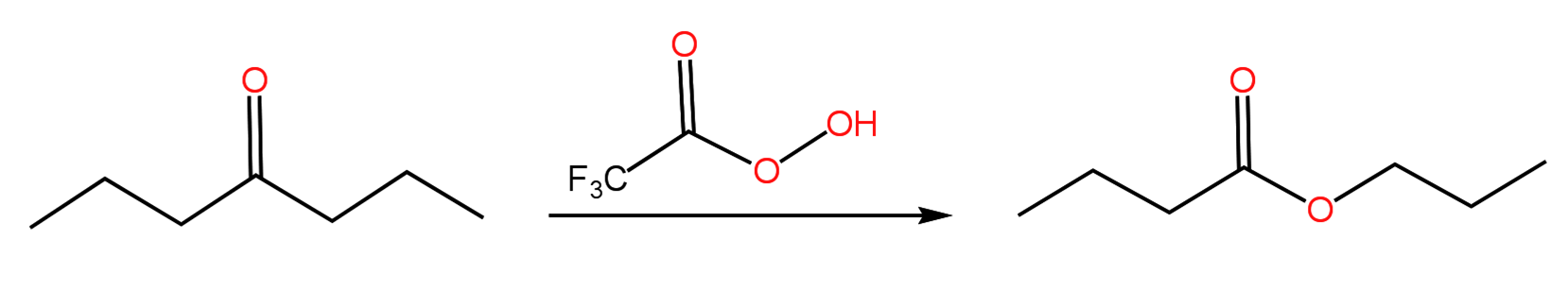
If the ketone is cyclic, a lactone (cyclic ester) is formed:
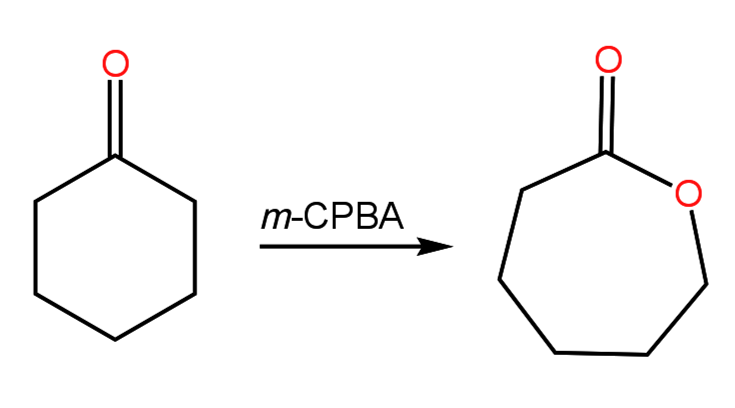
The mechanism involves the formation of an uncharged tetrahedral intermediate after attack of the protonated carbonyl by the peroxyacid. The carbonyl is reformed through migration of an alkyl group and subsequent deprotonation. The peroxyacid is converted to a carboxylic acid during the process. If the ketone is asymmetric, the reaction is regiospecific with respect to which group will migrate:
H > 3°C > 2°C, phenyl > 1°C > CH3
In other words, hydrogen will migrate faster than any other group, while methyl will migrate slower than any other group. The mechanism can be found below, along with practice problems.

