Guilford Techno Consultants, Inc
Drawing Newman Projections
Monday, January 9, 2023 by Guilford Techno Consultants, Inc. | Conformational Analysis
I use Newman projections often in OChem I. They are the best way to rotate around a carbon-carbon bond so I like to use them when I have to do a bond rotation on a starting material to get to the correct product in a reaction (for example, E2 elimination reactions, which will be discussed in another post). Many students find Newman projections difficult at first because it can be hard to visualize a three dimensional molecule drawn on a two dimensional page. Adding to the frustration is that organic chemists draw organic molecules as skeletal structures that do not depict hydrogen atoms.
A good perspective (wedge/dash) drawing is key to drawing a Newman projection correctly. Before I draw the projection, I make sure that all of the groups or atoms attached to each carbon of the bond I am sighting down are depicted. In other words, if I find that there are hydrogens missing from the structure, I draw them on the appropriate wedge or dash. It is important to remember that a wedge represents a group or atom coming out of the front of the page towards you, and a dash represents a group or atom going out the back of the page away from you. Any group or atom on a bond that is drawn as a straight line is in the plane of the page.
When sighting down the appropriate bond, I place myself either to the left or the right of the molecule, and then I draw exactly what I would see if I were physically in the plane of the page. Regardless of which side I am looking from, I draw the bonds that are on straight lines either up or down on the projection. If I am on the left hand side, that would put the bonds on wedges to my right and the bonds on dashes to my left, and that is how I would draw them on the projection. If I am on the right hand side, the bonds on wedges are on my left and the bonds on dashes are on my right. The example below illustrates my process for drawing a Newman projection. Take a look at it and convince yourself that each molecule would give the same Newman projection.
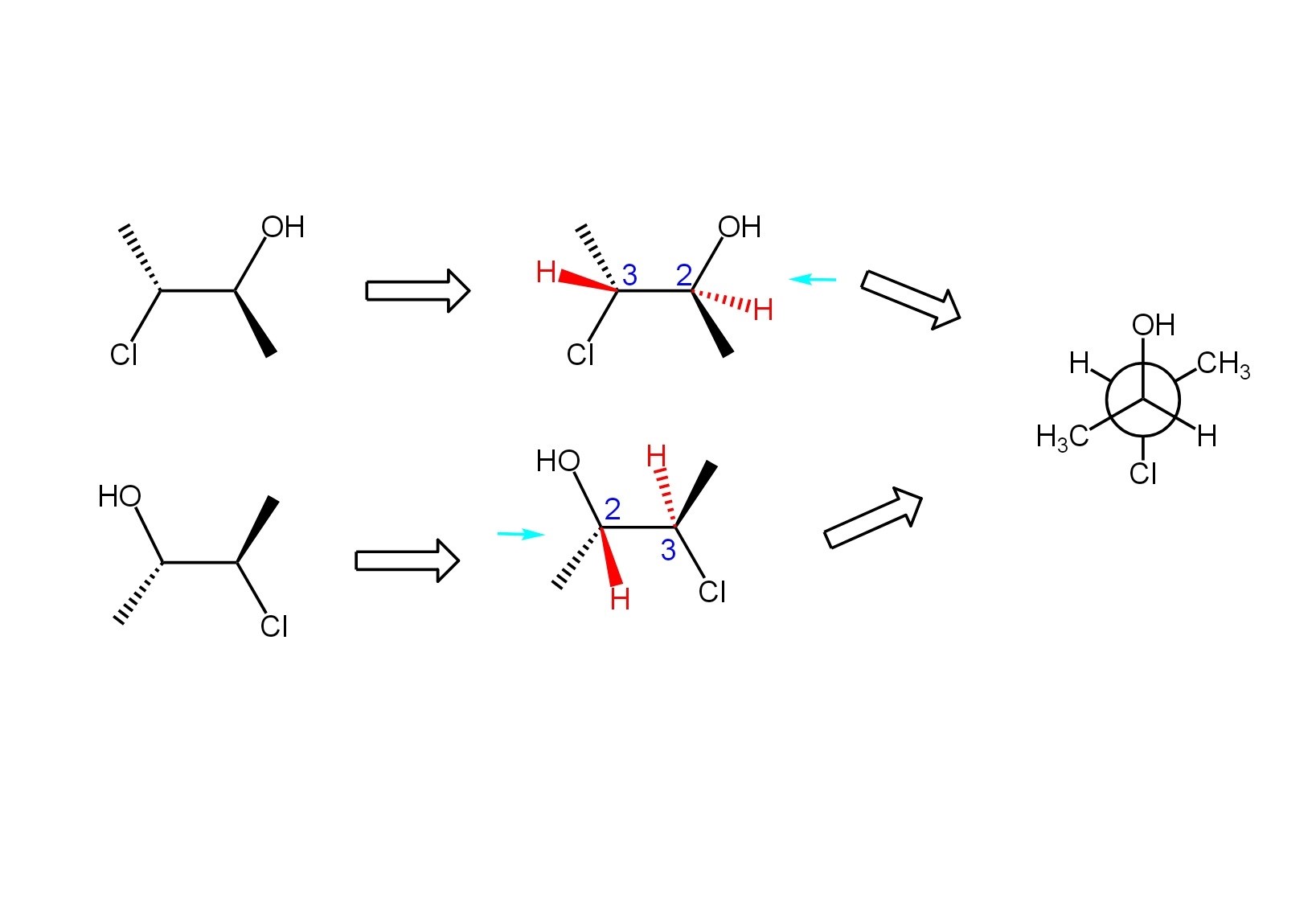
Practice problems can be found under the OChem I tab. For each molecule in the image, draw the corresponding Newman projection, sighting down the appropriate bond as indicated by the red arrow.

