Guilford Techno Consultants, Inc
Staudinger Reaction
Sunday, October 15, 2023 by Guilford Techno Consultants, Inc. | Name Reactions
The Staudinger reaction, discovered by Hermann Staudinger involves the reduction of organic azides by a phosphine to yield an amine. If triphenylphosphine is used, nitrogen gas and triphenylphosphine oxide are produced as by-products:
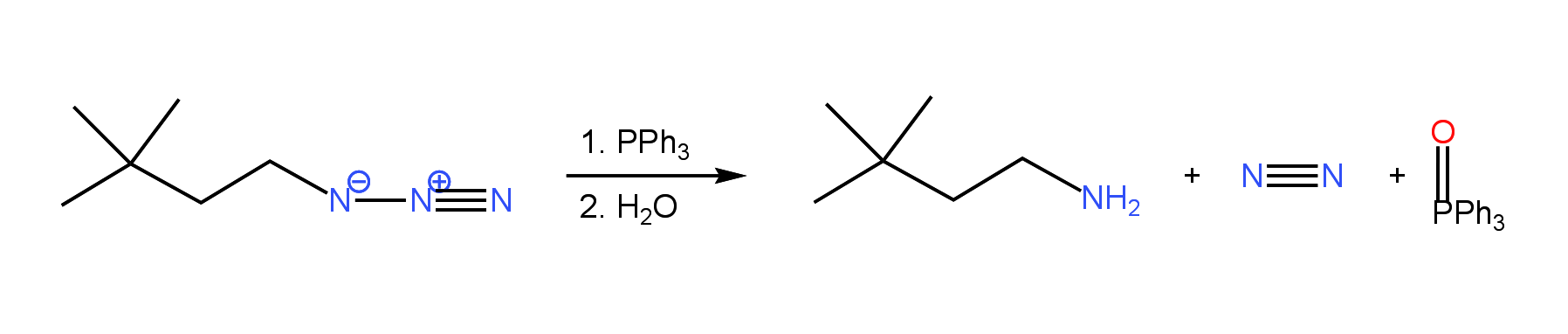
The reaction occurs in two steps. In the first step, the azide is reacted with triphenylphosphine to produce an iminophosphorane intermediate and nitrogen gas. Subsequent reaction of the iminophosphorane with water yields the desired amine product and triphenylphosphine oxide.
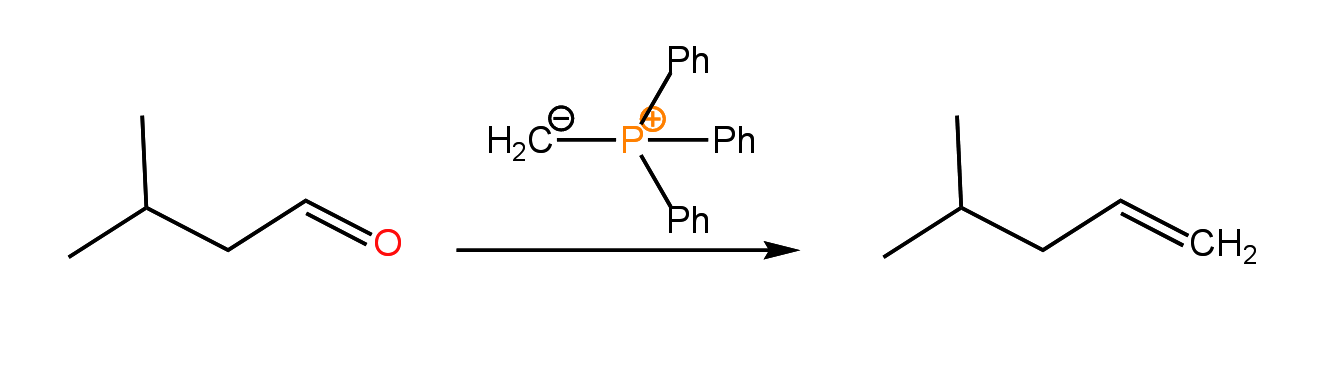
The Staudinger reaction is similar to the Wittig reaction in which an aldehyde or ketone is converted to an alkene through reaction with a phosphorous ylide:
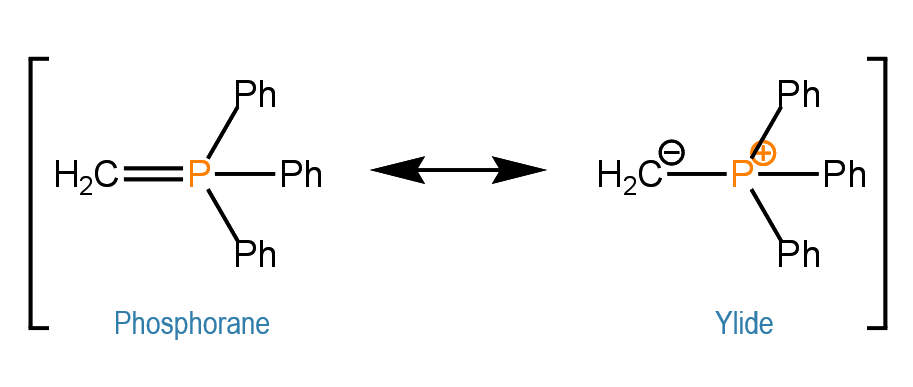
Phosphorous ylides, also known as Wittig reagents, are resonance stabilized carbanions:
The mechanism for the Staudinger reaction, along with practice problems, can be found in the following presentation.
Ozonolysis of Alkenes
Sunday, September 10, 2023 by Guilford Techno Consultants, Inc. | Alkene Reactions
For whatever reason, alkene reactions and their mechanisms are my favorite topics from the first semester. Although these reactions involve a large number of reagents and can be mechanistically complex, I find them much more straightforward than substitution and elimination reactions. From a mechanistic point of view, substitution and elimination reactions aren’t very complicated but there are so many moving parts to these reactions that I always feel like I’m guessing at what the products should be. Although there are quite a number of alkene reactions, I find product prediction to be fairly manageable. I also find alkene reaction mechanisms to be fairly intuitive with a few exceptions, ozonolysis being one of them.
The sigma and pi bonds of alkene double bonds can be oxidatively cleaved to yield two carbonyl groups as aldehydes or ketones depending on the structure of the alkene. One way of achieving this is through reaction of an alkene with ozone, O3, followed by a mild reducing step to yield product. Dimethyl sulfide (DMS) and zinc in water are commonly used as reductants. Initially, ozone adds to the alkene double bond to form a five-membered ring called a molozonide. The molozonide is unstable and rearranges through a two step process to yield an ozonide, which is then treated with a reductant to produce the final products. The mechanism for the formation of the ozonide and its reduction by DMS can be found in the following presentation. Practice problems are also included.
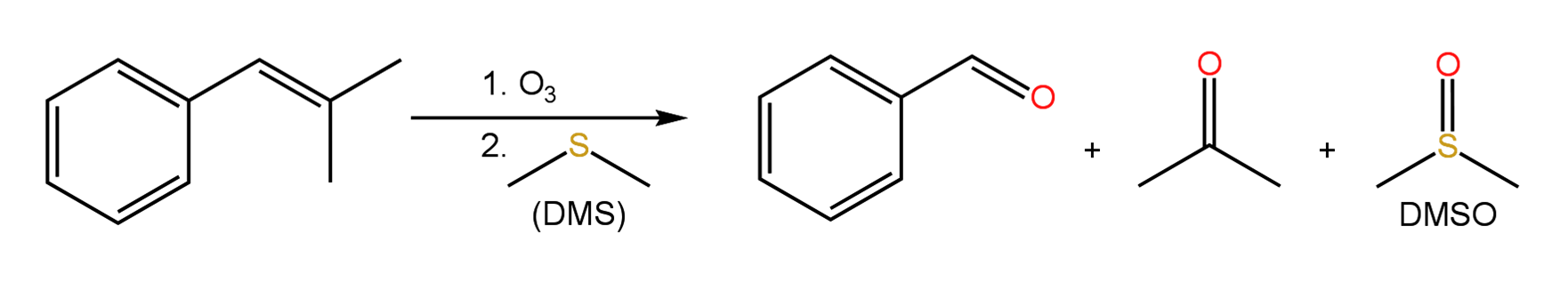
Click Here for Ozonolysis of Alkenes
Baeyer-Villiger Oxidation
Sunday, August 20, 2023 by Guilford Techno Consultants, Inc. | Name Reactions
Several mechanisms of the second semester of Organic Chemistry involve the migration (shift) of an alkyl group to give a rearranged product. The Hofmann rearrangement, which I wrote about in my last post, is one such mechanism. Another reaction that proceeds through an alkyl shift is the Baeyer-Villiger oxidation of a ketone by a peroxyacid to yield an ester. The reaction was discovered by Adolf von Baeyer and Victor Villiger in 1899 and involves the insertion of an oxygen atom of the peroxyacid in between the carbonyl of the ketone and one of it’s alkyl groups:
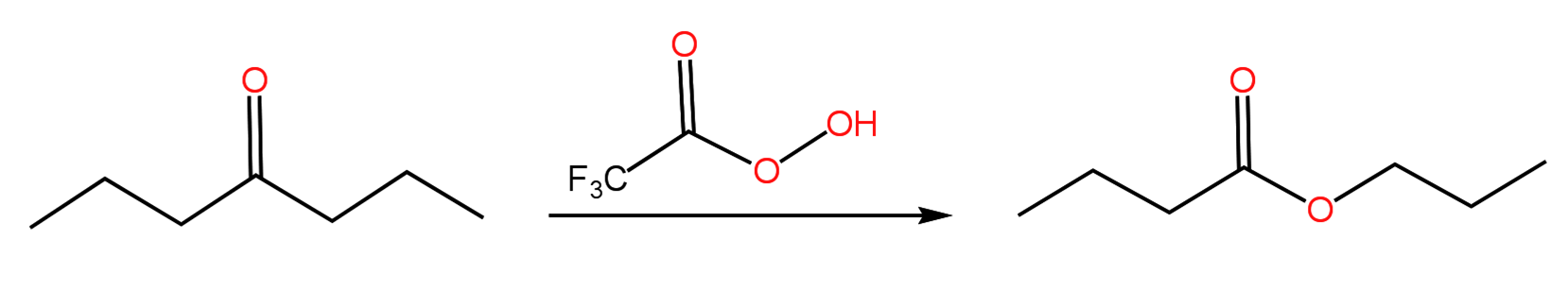
If the ketone is cyclic, a lactone (cyclic ester) is formed:
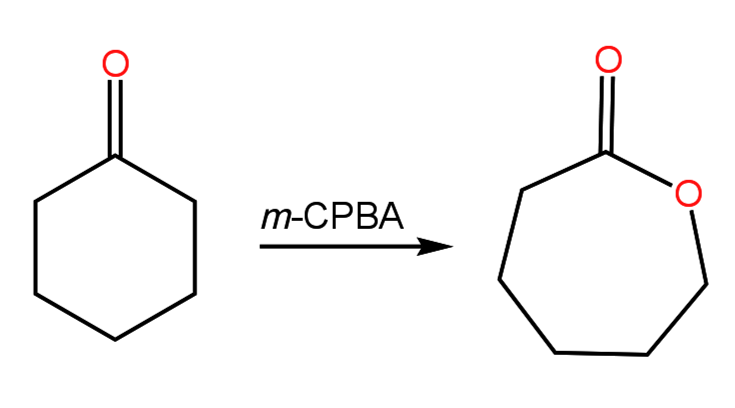
The mechanism involves the formation of an uncharged tetrahedral intermediate after attack of the protonated carbonyl by the peroxyacid. The carbonyl is reformed through migration of an alkyl group and subsequent deprotonation. The peroxyacid is converted to a carboxylic acid during the process. If the ketone is asymmetric, the reaction is regiospecific with respect to which group will migrate:
H > 3°C > 2°C, phenyl > 1°C > CH3
In other words, hydrogen will migrate faster than any other group, while methyl will migrate slower than any other group. The mechanism can be found below, along with practice problems.

