Guilford Techno Consultants, Inc
Cannizzaro Reaction
Thursday, May 25, 2023 by Guilford Techno Consultants, Inc. | Name Reactions
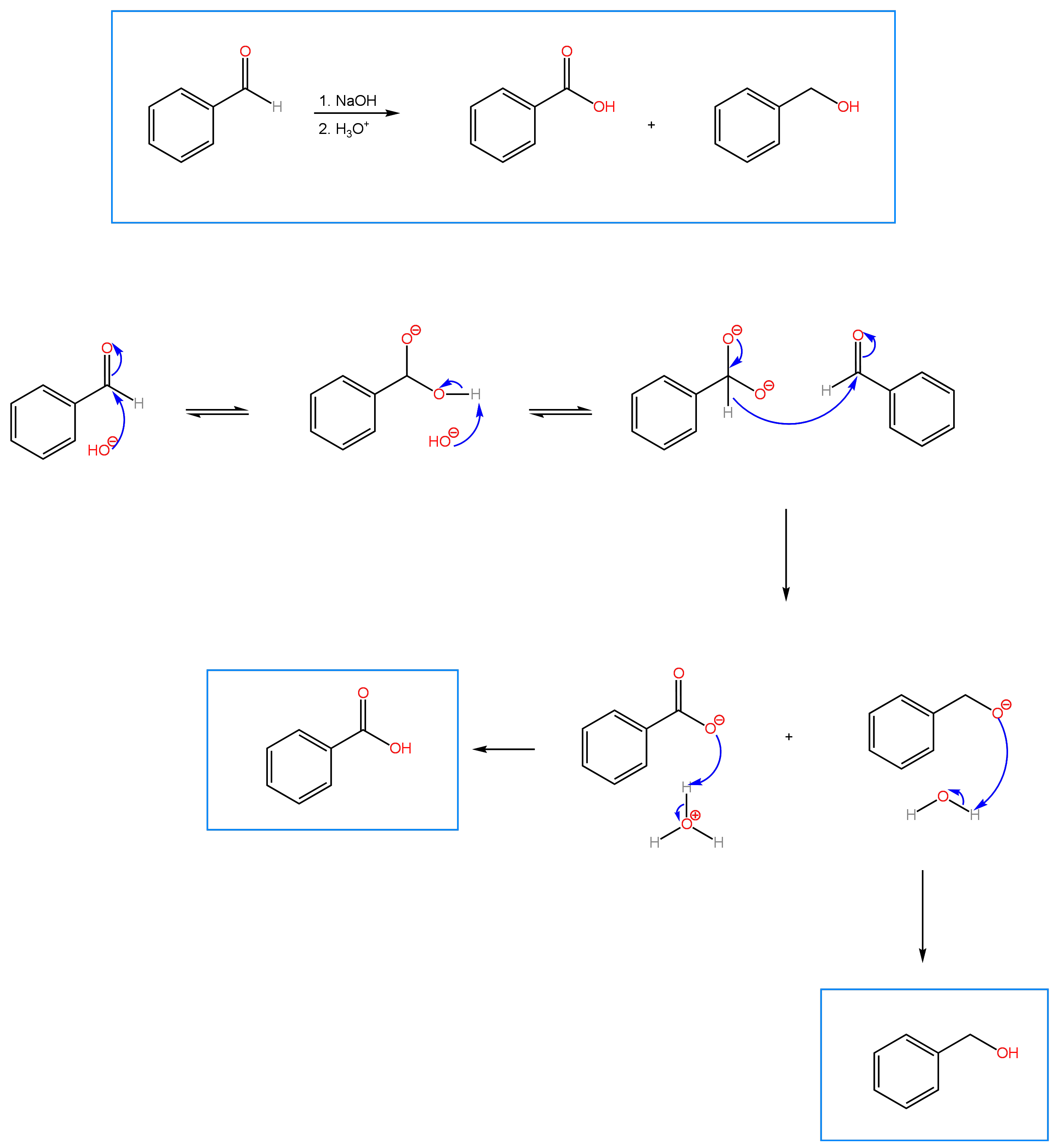
An interesting reaction that is sometimes covered in undergraduate Organic Chemistry is the Cannizzaro reaction, discovered by Stanislaus Cannizzaro and dating back to 1853. The reaction involves the disproportionation of a non-enolizable aldehyde under basic conditions to yield a carboxylic acid and an alcohol. Disproportionation refers to a redox reaction in which a molecule is simultaneously oxidized to one product (in this case, a carboxylic acid) and reduced to another (an alcohol). A non-enolizable aldehyde is an aldehyde that doesn’t contain an alpha carbon with acidic protons.
In terms of mechanism (found below), the reaction is straight forward. Nucleophilic acyl attack by the hydroxide anion on the carbonyl carbon of the aldehyde produces a tetrahedral intermediate. Deprotonation of the OH group gives a dianion, which reforms the carbonyl and causes a hydride to shift to the carbonyl carbon of a second mole of the aldehyde. The alkoxide formed from the hydride shift is protonated by water to give the alcohol. In a second acid step, the carboxylic acid is produced through protonation of the carboxylate. The Crossed Cannizzaro Reaction involves the reaction of an aldehyde with formaldehyde as reducing agent. Practice problems can be found under the OChem II tab. For each reaction, predict the product and propose a mechanism.