Guilford Techno Consultants, Inc
Strecker Amino Acid Synthesis
Thursday, December 28, 2023 by Guilford Techno Consultants, Inc. | Name Reactions
A large portion of the second semester of Organic Chemistry is spent learning about carbonyl chemistry which includes the reactions and mechanisms of aldehydes and ketones, carboxylic acids and their derivatives, as well as alpha carbon chemistry. The volume of material can seem a bit daunting. The situation isn’t helped by the fact that many of the reactions appear similar to each other. However overwhelming the material may appear to be, it plays a critical role in Biochemistry, in which carbonyl chemistry figures prominently in many processes.
The Strecker Amino Acid Synthesis, also known as the Strecker Synthesis, was discovered by Adolph Strecker. It can be used to convert an aldehyde to an alpha-amino acid:
In the first step of the synthesis, an aldehyde is converted to an iminium cation through reaction with ammonium chloride. Subsequent addition of cyanide anion to the iminium ion results in formation of an alpha-amino nitrile. Acid catalyzed hydrolysis of the nitrile group produces an alpha-amino acid. The Strecker Synthesis involves three important mechanisms of Organic Chemistry II: (1) Formation of an imine in the form of an iminium cation, (2) Addition of a cyanide anion to a carbon-nitrogen double bond (similar to cyanohydrin formation), and (3) Acid catalyzed hydrolysis of a nitrile. A mechanism for the Strecker Synthesis can be found in the following presentation.
Click Here for the Strecker Amino Acid Synthesis
Horner-Wadsworth-Emmons Reaction
Thursday, November 16, 2023 by Guilford Techno Consultants, Inc. | Name Reactions
A few of my students are learning about aldehydes and ketones in Organic Chemistry II. Carbonyl chemistry makes up a large portion of the second semester and can often be overwhelming because of the many reactions and mechanisms that must be learned. One of the reactions that I have always found interesting is the Wittig reaction, discovered by Georg Wittig, which involves the conversion of an aldehyde or ketone into an alkene through reaction of the carbonyl group with a resonance stabilized carbanion known as an ylide (Wittig reagent):
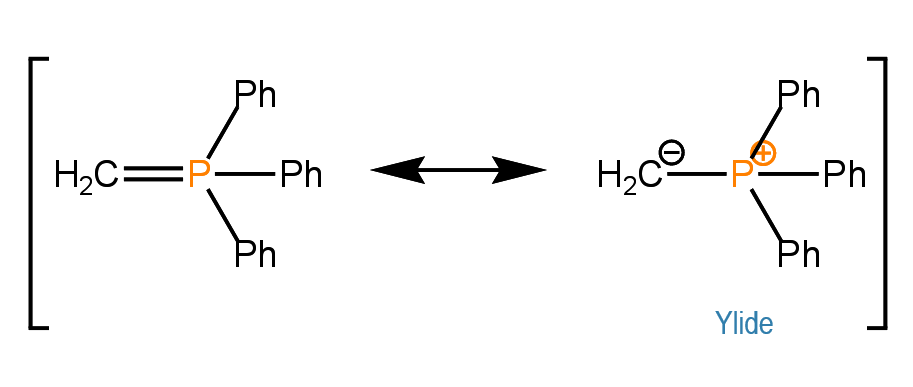
When the ylide contains a simple alkyl group, the reaction produces predominately the Z isomer of the alkene:
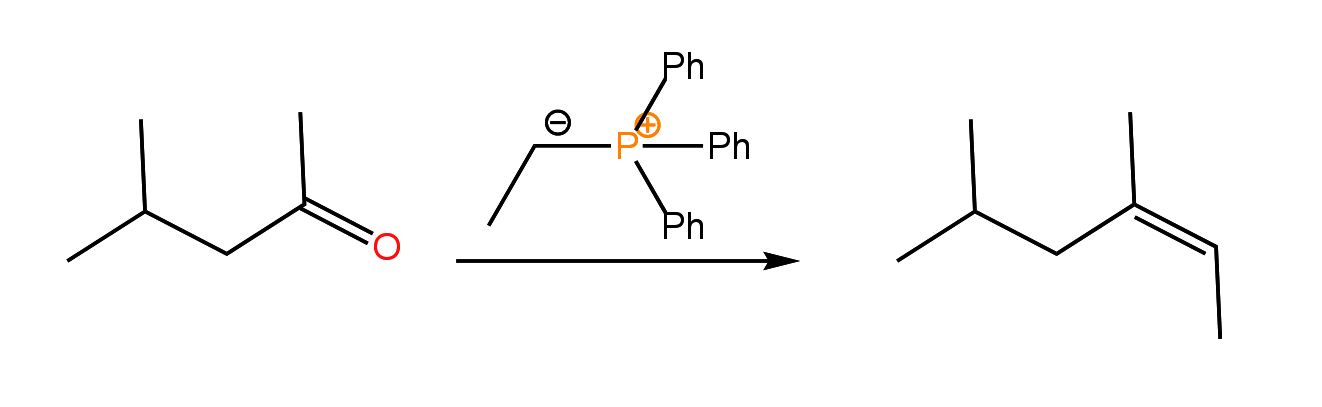
The ylide may, however, contain an electron withdrawing group, which stabilizes the reagent through an additional resonance structure:
Ylides containing electron withdrawing groups are referred to as stabilized Wittig reagents. These stabilized Wittig reagents afford mainly the E isomer of the alkene:
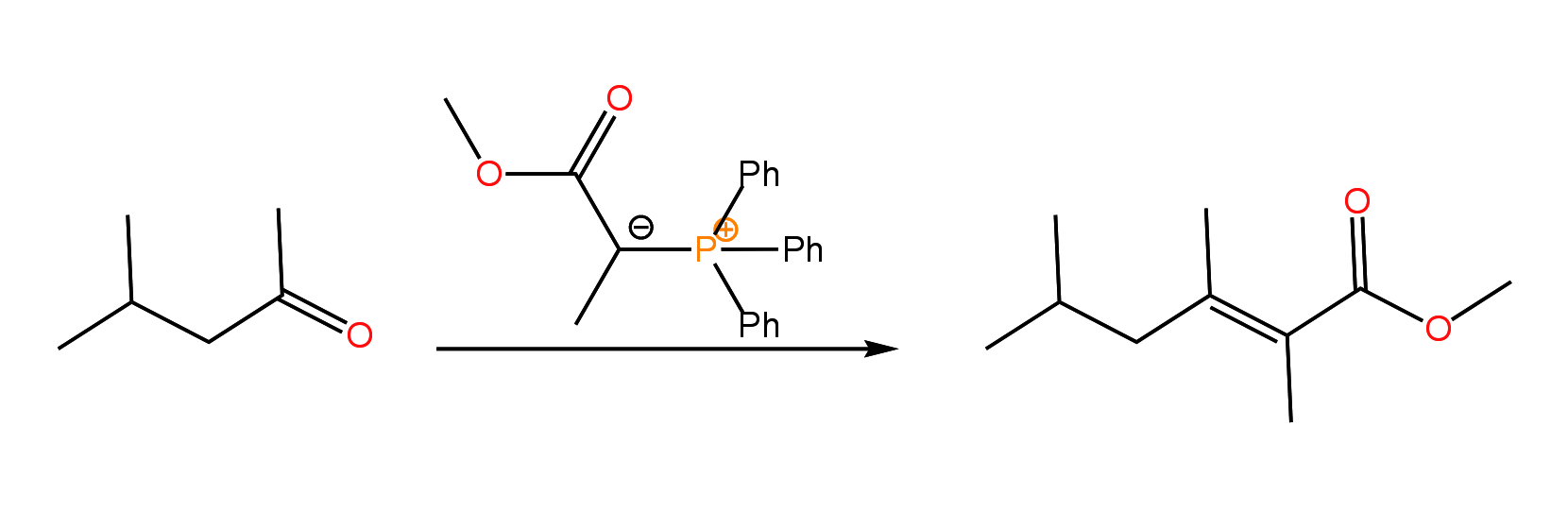
A variation of the Wittig reaction which is touched upon in the second semester is the Horner-Wadsworth-Emmons reaction in which a phosphonate ester carbanion (HWE reagent) is used in place of a stabilized Wittig reagent. Like the stabilized Wittig reagent, the HWE reagent produces the E isomer of the alkene as the major product:
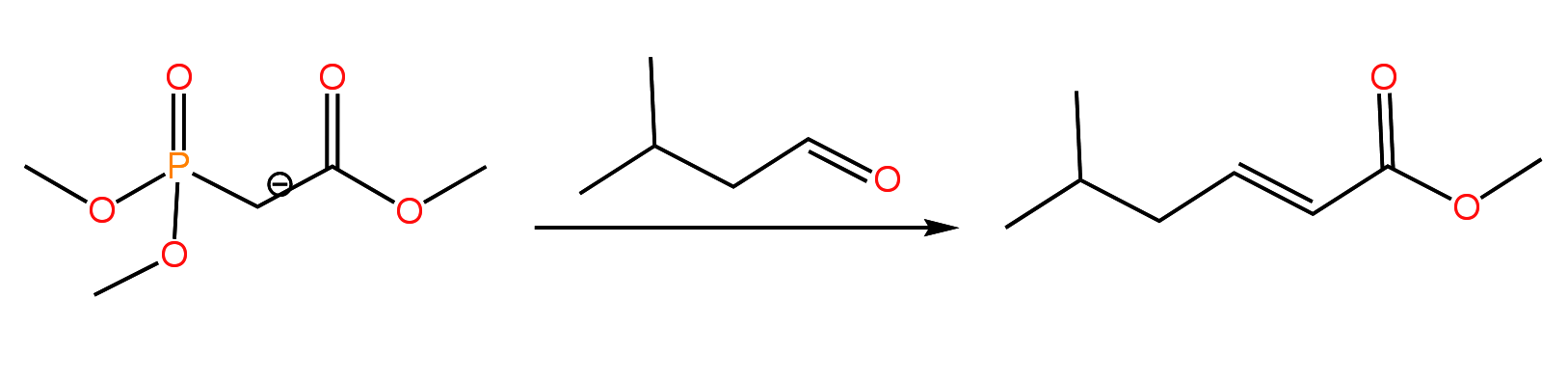
Both the Wittig and HWE reactions proceed through the fragmentation of an oxaphosphetane. I have included a mechanism for the HWE reaction in the following presentation, which also includes practice problems.
Click Here for the Horner-Wadsworth-Emmons Reaction
Staudinger Reaction
Sunday, October 15, 2023 by Guilford Techno Consultants, Inc. | Name Reactions
The Staudinger reaction, discovered by Hermann Staudinger involves the reduction of organic azides by a phosphine to yield an amine. If triphenylphosphine is used, nitrogen gas and triphenylphosphine oxide are produced as by-products:
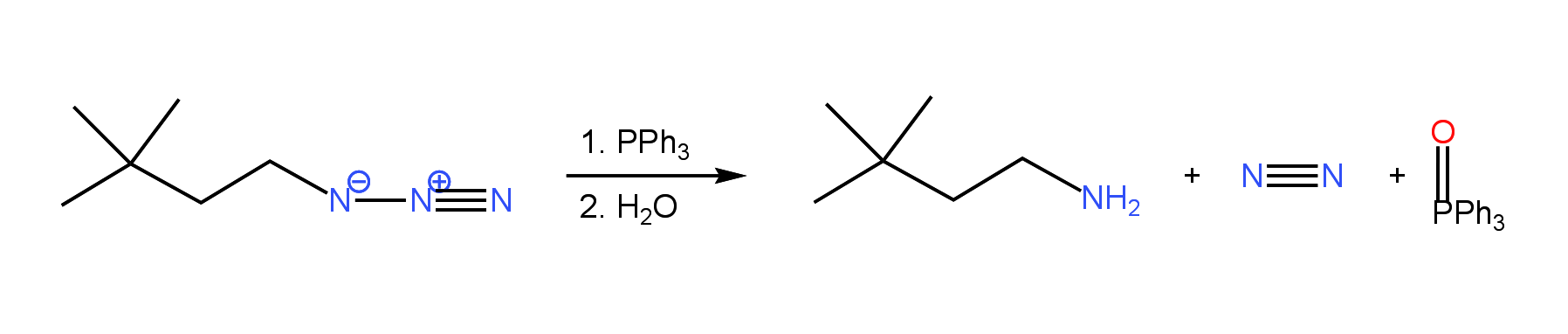
The reaction occurs in two steps. In the first step, the azide is reacted with triphenylphosphine to produce an iminophosphorane intermediate and nitrogen gas. Subsequent reaction of the iminophosphorane with water yields the desired amine product and triphenylphosphine oxide.
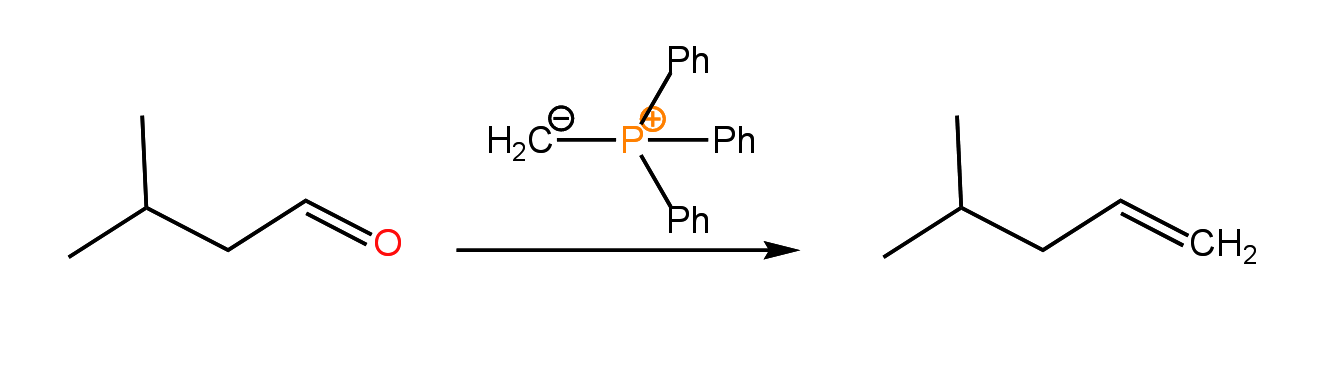
The Staudinger reaction is similar to the Wittig reaction in which an aldehyde or ketone is converted to an alkene through reaction with a phosphorous ylide:
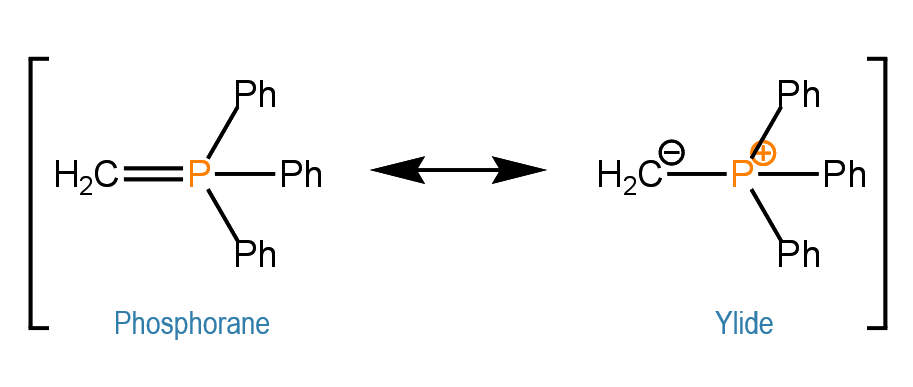
Phosphorous ylides, also known as Wittig reagents, are resonance stabilized carbanions:
The mechanism for the Staudinger reaction, along with practice problems, can be found in the following presentation.

